splashtech
New Member
- Joined
- Apr 27, 2011
- Messages
- 6
- Reaction score
- 0
Got a quick question I hope you might be able to advise me on... I have a new tank that's been up and running with 4 fish in a fish-in cycle (I know...) since the end of May. Been doing regular water tests and changes of course, but lately (last week or so), readings for ammonia, nitrite AND nitrate have all been high every single day necessitating daily large water changes - and the following day it's back. It seems to be going in the wrong direction. Any ideas what might be causing that? Haven't done anything to the filter or put any untreated tap water in, and can't figure it out.
To show the extent of the problem, yesterday I did what must have been an 80% or so water change and today the readings are back up to:
Ammonia: 0.4 (!!)
Nitrite: 0.25
Nitrate: 30
pH hovers between 7.5 and 7.6.
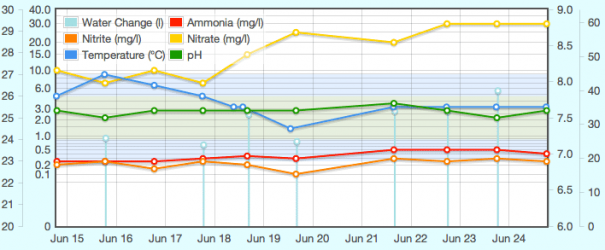
The attached graph shows the last 10 days. (the left-middle scale is the mg/l one, logarithmic so rises within the bottom stand out. The pH scale is the right-middle, and the temperature is left-outer. Right-outer scale is litres, for water changes. Misleading though as it's very much impossible to take all the water out of the gravel and whatnot.
Sooo... what do you make of this?
To show the extent of the problem, yesterday I did what must have been an 80% or so water change and today the readings are back up to:
Ammonia: 0.4 (!!)
Nitrite: 0.25
Nitrate: 30
pH hovers between 7.5 and 7.6.
The attached graph shows the last 10 days. (the left-middle scale is the mg/l one, logarithmic so rises within the bottom stand out. The pH scale is the right-middle, and the temperature is left-outer. Right-outer scale is litres, for water changes. Misleading though as it's very much impossible to take all the water out of the gravel and whatnot.
Sooo... what do you make of this?