🌟 Exclusive Amazon Black Friday Deals 2024 🌟
Don’t miss out on the best deals of the season! Shop now 🎁
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Rocks that turn water acidic
- Thread starter TNG
- Start date
I'm going to follow here. I don't know what they are, but they are interesting.
TNG
Fish Crazy
Hopefully, there's a geologist among us. My last geology lesson was decades ago, and I was never interested in it.
Igneous Rocks | Geological Society of Glasgow
Igneous rocks are formed when molten material from within the Earth, called magma, cools down and solidifies forming crystals.
I just learned something.... see if this helps.
This is one of those things that are very difficult to impossible to tell from a picture but I see lichen on one of those rocks . That shouldn’t turn your water acidic but if these rocks were not washed prior to putting them in your aquarium then they might have rodent urine on them that would . Rodents eat lichen and mark their territory .
I’ve never witnessed a rock that would effect the ph to acid, in an aquarium, that gets water changes as recommended… so maybe in a stagnant pool, but not that would leach anything into the water fast enough to effect the ph in an aquarium… not saying it can’t happen, as I’ve not put every type of rock into an aquarium, but I’ve never seen even pure lead be a problem in a tank that gets recommended water changes… I have seen “basic” changes using crushed coral or lime stone as gravel, probably because of the softer mineral, and increased surface area
TwoTankAmin
Fish Connoisseur
Rocks that are soft, you can easily scratch them or break them are normally the ones which will increase pH. They do this because the water is acid and it will dissolve the rock. We can test rocks to determine if they might increase pH using some form of acid. I have Muriatic acid I use for lowering the pH in one tank, so I would use that. But it is strong stuff and not for inexperienced users. However, vinegar is quite acid and can be used.
Put a bit of the acid/vinegar onto the rocks, If the result is fizzing, you know that rock can raise the pH. The more ecid the water is, he faster it will dissolved the rock. If we want to raise the pH in a tank we must raise th KH. In tanks this is mostly caused by carbonates and bicarbonates. So we are not limited to rocks for increasing the KH, and even the GH in some cases, we can use crushed coral, shells etc. Anything containing or made from these things will be dissolved by acid and then work to increse pH.
Making water more acid involves the reverse process. One need to lower the KH.
edited to fix typos
Put a bit of the acid/vinegar onto the rocks, If the result is fizzing, you know that rock can raise the pH. The more ecid the water is, he faster it will dissolved the rock. If we want to raise the pH in a tank we must raise th KH. In tanks this is mostly caused by carbonates and bicarbonates. So we are not limited to rocks for increasing the KH, and even the GH in some cases, we can use crushed coral, shells etc. Anything containing or made from these things will be dissolved by acid and then work to increse pH.
Making water more acid involves the reverse process. One need to lower the KH.
Buffering Capacity (KH, Alkalinity)
Buffering capacity refers to water's ability to keep the pH stable as acids or bases are added. pH and buffering capacity are intertwined with one another; although one might think that adding equal volumes of an acid and neutral water would result in a pH halfway in between, this rarely happens in practice. If the water has sufficient buffering capacity, the buffering capacity can absorb and neutralize the added acid without significantly changing the pH. Conceptually, a buffer acts somewhat like a large sponge. As more acid is added, the ``sponge'' absorbs the acid without changing the pH much. The ``sponge's'' capacity is limited however; once the buffering capacity is used up, the pH changes more rapidly as acids are added.
Buffering has both positive and negative consequences. On the plus side, the nitrogen cycle produces nitric acid (nitrate). Without buffering, your tank's pH would drop over time (a bad thing). With sufficient buffering, the pH stays stable (a good thing). On the negative side, hard tap water often almost always has a large buffering capacity. If the pH of the water is too high for your fish, the buffering capacity makes it difficult to lower the pH to a more appropriate value. Naive attempts to change the pH of water usually fail because buffering effects are ignored.
In freshwater aquariums, most of water's buffering capacity is due to carbonates and bicarbonates. Thus, the terms ``carbonate hardness'' (KH), ``alkalinity'' and ``buffering capacity'' are used interchangeably. Although technically not the same things, they are equivalent in practice in the context of fishkeeping. Note: the term ``alkalinity'' should not be confused with the term ``alkaline''. Alkalinity refers to buffering, while alkaline refers to a solution that is a base (i.e., pH > 7).
edited to fix typos
Last edited:
I have no experience, and think I noticed this in passing, but would coal not acidify water?It seems to me I read someone's story of doing this. A quick google search talks of mining effluents and such, but there isn't much on dropping a lump of coal into a fishtank.
TNG
Fish Crazy
This is one of those things that are very difficult to impossible to tell from a picture but I see lichen on one of those rocks . That shouldn’t turn your water acidic but if these rocks were not washed prior to putting them in your aquarium then they might have rodent urine on them that would . Rodents eat lichen and mark their territory .
I agree, it’s difficult to tell from photos. Good observation on the lichen.
The rocks were from a mountain around 1500m in elevation. It’s cool and moist most of the year with snow in winter. The only rodents in that part of the state are mice but they (and other animals) stay at much lower elevation to keep warm, so we can rule out rodents’ territory marking. I gave the rocks a good wash anyway.
I’ll see if I can find a geological map to tell the type of rocks and the mineral content.
Rocks that are soft, you can easily scratch them or break them are normally the ones which will increase pH. They do this because the water is acid and it will dissolve the rock. We can test rocks to determine if they might increase pH using some form of acid. I have Muriatic acid I use for lowering the pH in one tank, so I would use that. But it is strong stuff and not for inexperiencced users. However, vinegar is quite acid and can be used.
Put a bi if the acid/vinegar onto the rocks, If the result is fizzing, you know that rock can raise the pH. The more ecid the water is, he faster it will dissolved the rock. If we want to raise the pH in a tank we must raise th KH. In tanks this is mostly caused by carbonates and bicarbonates. So we are not limited to rocks for increasing the KH, and even the GH in some cases, we can use crushed coral, shells etc. Anything containing or made from these things will be dissolved by acid and then work to increse pH.
Making water more acid involves the reverse process. One need to lower the KH.
Thanks for the link. The KH of my tap water is low, so it’s not unexpected the pH drops. Using rocks seem like a safe way to lower pH as drift wood and almond leaf don’t do much – not that I have a need to do it as my tap water is soft enough. I'll see if l can find out the mineral composition, just out of interest.
Uberhoust
Fish Herder
Your rocks look like they have some iron staining in them, suggesting they are not simply dolomite (magnesium carbonate). If there are any sulphide minerals in them on exposure to moisture they will release acid. This is one of the major sources of acid runoff from mine tailings. Most of the time we collect rocks that have been on the surface for some time but if these rocks are from a mine they may contain minerals normally not found un-oxidized on the surface. More history on the rocks might clear up what is happening.
Uberhoust
Fish Herder
Found a brief paper on acid runoff from mine tailings here. https://uwaterloo.ca/wat-on-earth/n...erials are exposed to atmospheric oxygen (O2).
Dolomite is CaMg(CO3)2 There is noting in dolomite that would make water. Generally dolomite react with acid releasing CO2 and that pushes the PH up to about 7. Once the PH is above 7 the dolomite stops reacting and PH stabilizes at 7 or very close to it. The CO2 released can make the water acidic but but in my experience it is hard to get enough CO2 in a tank to drop the PH that much. You would basically need a prssurizeed CO2 system to do that. In any case after a few days in the glass the CO2 should outgas and the PH may change.
Looking at your pictures I cannot tell what those rocks are. But if you expose the rock to vinegar and it fizzes it is probably a carbonate rock of some kind. If it does not it is something else.
Mine tailing runoff is often the result of sulfide minerals reacting with water and are resoling in metal oxides and sulfuric acid which often lead he's other minerals and sulfur out of the rock. SO2 can easily reduce the PH to 6 or less. With enough a PH below 4 is possible.
Looking at your pictures I cannot tell what those rocks are. But if you expose the rock to vinegar and it fizzes it is probably a carbonate rock of some kind. If it does not it is something else.
Mine tailing runoff is often the result of sulfide minerals reacting with water and are resoling in metal oxides and sulfuric acid which often lead he's other minerals and sulfur out of the rock. SO2 can easily reduce the PH to 6 or less. With enough a PH below 4 is possible.
TNG
Fish Crazy
Found a brief paper on acid runoff from mine tailings here. https/uwaterloo.ca/wat-on-earth/news/acid-mine-drainage-past-presentfuture#:~:text=The generation of acid mine drainage (AMD) and,these materials are exposed to atmospheric oxygen (O2).
Dolomite is CaMg(CO3)2 There is noting in dolomite that would make water. Generally dolomite react with acid releasing CO2 and that pushes the PH up to about 7. Once the PH is above 7 the dolomite stops reacting and PH stabilizes at 7 or very close to it. The CO2 released can make the water acidic but but in my experience it is hard to get enough CO2 in a tank to drop the PH that much. You would basically need a prssurizeed CO2 system to do that. In any case after a few days in the glass the CO2 should outgas and the PH may change.
Looking at your pictures I cannot tell what those rocks are. But if you expose the rock to vinegar and it fizzes it is probably a carbonate rock of some kind. If it does not it is something else.
Mine tailing runoff is often the result of sulfide minerals reacting with water and are resoling in metal oxides and sulfuric acid which often lead he's other minerals and sulfur out of the rock. SO2 can easily reduce the PH to 6 or less. With enough a PH below 4 is possible.
Thanks for your input. Much appreciated.
I don’t disagree with what everyone says about the mine tailing runoff, but there’s no mining anywhere in the area.
The outcrop at the bottom left corner of photo 1 is very much similar to where the rocks were collected. The other 2 photos are the close-up using a macro lens 1:1 and each rock is about 4” or 100mm across.
Vinegar tests say they’re not carbonate rock.
Next time in the area I’ll collect from a few different outcrops and see if they all lower the pH, just out of curiosity.
Attachments
Uberhoust
Fish Herder
There is still what appears to be iron staining on the rocks, and they appear sedentary but I wouldn't bet the farm on that statement. I don't think I can ID the rocks without some concept of what to expect in the area, and I don't know the basics of the general geology in Australia, other than it must be interesting given all the rocks that come from there.
Getting to your issue of the PH dropping, if it is an issue.
1. The rocks may be the cause. To test simply place them in water aged a day, and monitor the pH.
2. The rocks are not the cause. It is possible your tap water has been adjusted for pH and it is stabilizing after it comes out of the tap, the normal pH is 6 but is pushed higher as a water treatment to reduce corrosion on the community water supply.
3. The rocks are not the cause but something else is causing the drop in pH, maybe some tannic or humic acids introduced with the substrate, wood, or other additions to the tank.
A closer location would allow us to look at surface geology maps, of the area.
Just a thought is that pyrite is pretty common in some sedimentary sourced rocks. If you look at the rocks with a magnifying glass do you see any cubic shaped small crystals? Typically these should already be weathered out but maybe you have some fresh faces on the rocks that you collected. Pyrite can cause acidification.
Getting to your issue of the PH dropping, if it is an issue.
1. The rocks may be the cause. To test simply place them in water aged a day, and monitor the pH.
2. The rocks are not the cause. It is possible your tap water has been adjusted for pH and it is stabilizing after it comes out of the tap, the normal pH is 6 but is pushed higher as a water treatment to reduce corrosion on the community water supply.
3. The rocks are not the cause but something else is causing the drop in pH, maybe some tannic or humic acids introduced with the substrate, wood, or other additions to the tank.
A closer location would allow us to look at surface geology maps, of the area.
Just a thought is that pyrite is pretty common in some sedimentary sourced rocks. If you look at the rocks with a magnifying glass do you see any cubic shaped small crystals? Typically these should already be weathered out but maybe you have some fresh faces on the rocks that you collected. Pyrite can cause acidification.
TNG
Fish Crazy
There is still what appears to be iron staining on the rocks, and they appear sedentary but I wouldn't bet the farm on that statement. I don't think I can ID the rocks without some concept of what to expect in the area, and I don't know the basics of the general geology in Australia, other than it must be interesting given all the rocks that come from there.
Getting to your issue of the PH dropping, if it is an issue.
1. The rocks may be the cause. To test simply place them in water aged a day, and monitor the pH.
2. The rocks are not the cause. It is possible your tap water has been adjusted for pH and it is stabilizing after it comes out of the tap, the normal pH is 6 but is pushed higher as a water treatment to reduce corrosion on the community water supply.
3. The rocks are not the cause but something else is causing the drop in pH, maybe some tannic or humic acids introduced with the substrate, wood, or other additions to the tank.
A closer location would allow us to look at surface geology maps, of the area.
Just a thought is that pyrite is pretty common in some sedimentary sourced rocks. If you look at the rocks with a magnifying glass do you see any cubic shaped small crystals? Typically these should already be weathered out but maybe you have some fresh faces on the rocks that you collected. Pyrite can cause acidification.
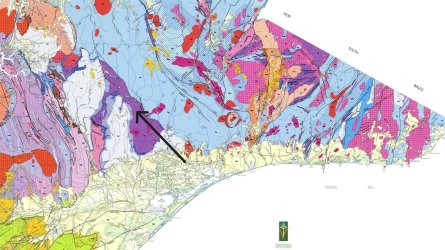
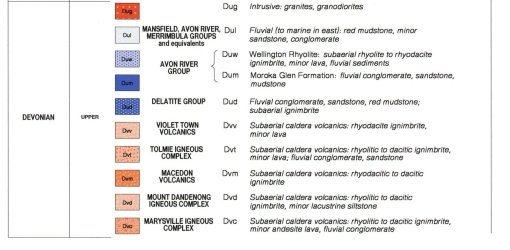
Attached is a geology map of eastern part of Victoria. The file is too large so I cropped the relevant section.
Tip of arrow is roughly the rock collection location. Zoom in on the arrow to see the code Sy for the purple area, with the adjacent areas being SDg & Duw.
Attachments ID1 & ID2 provide description of the rocks, although there’s nothing to suggest that they contain minerals that cause acidification.
Btw, no traces of pyrite on any of the collected rocks.
There’s no problem with water in the aquarium, it’s stable around pH 6.8 & 7.2, just where I want it to be. Tap water is roughly 6.8 after sitting in a plastic container for 24 – 48 hrs. Drop the rocks in it, and it drops to 6 or lower after 24 – 48hrs.
Anyway, I’ll leave it at that, but will collect a few more rocks for testing next time I’m in the area.
Attachments
Similar threads
- Replies
- 36
- Views
- 1K
- Replies
- 4
- Views
- 252