The problem is due to using stability.
The bacteria needed to handle ammonia and nitrite do not form spores. They reproduce by dividing. Stability is a bottle of spores. Tha co,bime with the info on SeaChem's page being mostly BS. Ther is a reason for this.
Note: I am quoting a couple of scientific papers below. You can skip them and just read what I wrote about them.
The most important bacteria we developed for cycling a tank are strains of Nitrospira. These were initially identified by Dr. Hovanec and associated scientists and revealed in this published, peer reviewed paper:
Nitrospira-Like Bacteria Associated with Nitrite Oxidation in Freshwater Aquaria
TIMOTHY A. HOVANEC, LANCE T. TAYLOR, ANDREW BLAKIS, AND EDWARD F. DELONG
Department of Ecology, Evolution and Marine Biology, University of California, Santa Barbara, Santa Barbara, California 93106,1 and Aquaria Inc., Moorpark, California 930212 Received 4 September 1997/Accepted 27 October 1997
Abstract
Oxidation of nitrite to nitrate in aquaria is typically attributed to bacteria belonging to the genus Nitrobacter which are members of the a subdivision of the class Proteobacteria. In order to identify bacteria responsible for nitrite oxidation in aquaria, clone libraries of rRNA genes were developed from biofilms of several freshwater aquaria. Analysis of the rDNA libraries, along with results from denaturing gradient gel electrophoresis (DGGE) on frequently sampled biofilms, indicated the presence of putative nitrite-oxidizing bacteria closely
related to other members of the genus Nitrospira. Nucleic acid hybridization experiments with rRNA from biofilms of freshwater aquaria demonstrated that Nitrospira-like rRNA comprised nearly 5% of the rRNA extracted from the biofilms during the establishment of nitrification. Nitrite-oxidizing bacteria belonging to the a subdivision of the class Proteobacteria (e.g., Nitrobacter spp.) were not detected in these samples. Aquaria which received a commercial preparation containing Nitrobacter species did not show evidence of Nitrobacter
growth and development but did develop substantial populations of Nitrospira-like species. Time series analysis of rDNA phylotypes on aquaria biofilms by DGGE, combined with nitrite and nitrate analysis, showed a correspondence between the appearance of Nitrospira-like bacterial ribosomal DNA and the initiation of nitrite oxidation. In total, the data suggest that Nitrobacter winogradskyi and close relatives were not the dominant nitrite-oxidizing bacteria in freshwater aquaria. Instead, nitrite oxidation in freshwater aquaria appeared to be mediated by bacteria closely related to Nitrospira moscoviensis and Nitrospira marina.
Pdf of the full paper:
Nitrospira-Like Bacteria Associated with Nitrite Oxidation in Freshwater Aquaria
However, this is not the end of the Nitrospira research which is why it is really the most important nacteria we colonize when we cycle a tank.
Daims, H., Lebedeva, E., Pjevac, P.
et al. Complete nitrification by
Nitrospira bacteria.
Nature 528, 504–509 (2015).
https://doi.org/10.1038/nature16461
Abstract
Nitrification, the oxidation of ammonia via nitrite to nitrate, has always been considered to be a two-step process catalysed by chemolithoautotrophic microorganisms oxidizing either ammonia or nitrite. No known nitrifier carries out both steps, although complete nitrification should be energetically advantageous. This functional separation has puzzled microbiologists for a century. Here we report on the discovery and cultivation of a completely nitrifying bacterium from the genus
Nitrospira, a globally distributed group of nitrite oxidizers. The genome of this chemolithoautotrophic organism encodes the pathways both for ammonia and nitrite oxidation, which are concomitantly activated during growth by ammonia oxidation to nitrate. Genes affiliated with the phylogenetically distinct ammonia monooxygenase and hydroxylamine dehydrogenase genes of
Nitrospira are present in many environments and were retrieved on
Nitrospira-contigs in new metagenomes from engineered systems.
These findings fundamentally change our picture of nitrification and point to completely nitrifying Nitrospira as key components of nitrogen-cycling microbial communities
Red color above added by me. The full paper is available here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5152751/
The result of the Hovanec paper above was that Dr. Hovanec and his employer at the time - the Aquaria Inc. Group of companies, including Marineland Labs, shared a patent for the bacteria and the method for detecting and using them. Dr. Tim left Aquaria Inc. when it was acquired by the Global Pet Care division of Spectrum Brands which owns fish related Tetra, Marineland, Glo Fish, Instant Ocean and Omega One.
Spectrum transfered the responsibility for using the patent to Tetra who then created Safe Start and Safe Start+ which also contain the Nitrospira bacteria. No other company can use Nitrospira in their product as a result.
So, Stability is out in the cold for two reasons.
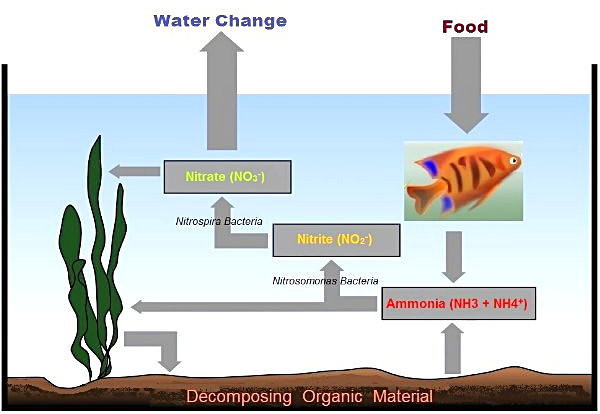
Why you have the problem with nitrite is explained by the info above. When we use either One and Only or Safe Start we are adding both the ammonia bacteria and the nitrite ones at the same time. What this means is that whatever amount of ammonia the bacteria are able to convert to nitrite, there are nitrite bacteria present which can convert that amount of nitrite to nitrate right away.
However, if we do a normal fishless cycle without and seeding of bacteria, the ammonia ones colonize first as we add ammonia to the tank. But for the nitrite ones to colonize there has to be nitrite. The Nitrospira reproduce more slowly then and ammonia only bacteria which is another reason why things need to wait for nitrite to become established when we can not seed the needed bacteria.
All of the above explain why you have elevated nitrite. Btw, when high enough, nitire is usually the cause of stalling a cycle. If we have more than 6.4 ppm of ammonia on an API test kit or more than 16.4 ppm of nitrite, it stalls the cycle. Also there is really no need to dose more than 3 ppm of ammonia to accomplish a fishless cycle.
Next, there are two scales used for testing for nitrogen compounds.
There are two major ways to describe the concentrations of ammonia, nitrite, and nitrate in water. The "nitrogen" weight of these molecules describes the weight of only the nitrogen atoms within them. On the other hand, the "ion" weight of these molecules describes the weight of the entire molecule.
For example, the term nitrate-nitrogen (NO3-N) refers to the weight of only the nitrogen atom within the nitrate molecule; as opposed to nitrate-ion (NO3), which describes the weight of the entire nitrate molecule. Note that a given nitrate-nitrogen value will always be lower than the associated nitrate-ion value. Conversion between the two forms is as simple as applying a constant..........
Scientific literature often uses the "nitrogen" form rather than the "ion" form to describe the concentration for these molecules.
from
https://www.hamzasreef.com/Contents/Calculators/NitrogenIonConversion.php
This explains why on Dr. Hovenec's site when he states that ammonia or nitrite should not exceed 5 ppm for cycling, he is using the Nitrogen scale. The numbers I provided for these two things earlier converted them to the Total Ion Scale from the nitrogen scale as most hobby kits use the Total Ion scale.
So the above explains exactly why you have high nitrite after adding Stability. There are only spores in it and that means no true nitrifying bacteria and most certainly no Ntrospira. But SeaChem is not the only outfit selling a product which doesn't contain Nitrospira. But other companies use another nitrite ooxidizing bacteria called Nitrobacter wynogradsky. This is named after Sergio Wynogradsky who
made a number of important discoveries and is considered the father of sulfur microbiology. From studying sulfur and nitrogen dependent microbes, Winogradsky was able to deduce that they obtained energy from chemical reactions and used that energy to grow on carbon dioxide, a process called chemoautotrophy.
The problem is this nitrite oxidizing bacteria thrives on much higher nitrite concentrations than we have in our tanks. The bacteria are the major one for handling the really high nitrite levels in waste water treatment.
So you now know more than you asked for as to why you have high nitrite and why so few products sold for cycling tanks actually do so. But companies who make products for our hobby needed to come up with something could sell and could state if helped even if it did not really do so as well. At least the ones with the live nitrobacter in them do initially help some with nitrite. Bu, when a tank is finally cycled, the Nitrobacter are mostly gone having been replaced by the needed Nitrospira.
When we cycle a tank without using bottled bacteria, we are getting the nitrospira which exist in mature and which find their way into our tanks from our water supply or even from the air. The cultured ones can be patented but the ones in nature cannot be.