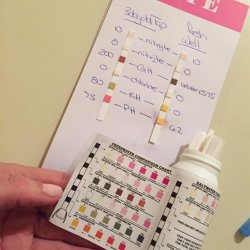
I see something here which I cannot explain, maybe Steven can. I am wondering why the GH and KH of the source water is so much lower in the aquarium. The source water appears to be very hard (300 ppm = 16 dGH), and in the aquarium it is soft (75 ppm = 4 dGH). I cannot fathom any way this could occur in the tank. Biological processes and plants cannot possibly remove this much mineral in weeks or months. Which makes me wonder what is the source of the high GH. The fact that the pH seems so ready to lower parallels the lower GH/KH which makes sense, but that doesn't explain the GH/KH aspect.
On the KH, this is not strictly speaking your issue, but it is a part of the overall issue. And yes, hardness is the amount of dissolved mineral in the water, primarily calcium and magnesium plus sometimes a few others but they are minimal. The KH is the amount of carbonate and bicarbonate ions in the water, and these ions are the salts of carbonic acid. This is why the KH acts to "buffer" pH, preventing it from fluctuating from the level it is at in the water to start with.
On the KH, this is not strictly speaking your issue, but it is a part of the overall issue. And yes, hardness is the amount of dissolved mineral in the water, primarily calcium and magnesium plus sometimes a few others but they are minimal. The KH is the amount of carbonate and bicarbonate ions in the water, and these ions are the salts of carbonic acid. This is why the KH acts to "buffer" pH, preventing it from fluctuating from the level it is at in the water to start with.


 /www.aquariumcenter.biz/FreshFish.html
/www.aquariumcenter.biz/FreshFish.html