Of the 14 minerals that plants need to thrive, it sounds like iron is the most unstable & the most important one that needs to be supplemented. Can I then assume that my water naturally has enough of the other minerals? And if I'm doing regular water changes, then these minerals levels are being adequately maintained?
Unfortunately no. Actually most of the micros are unstable (iron, manganese, zinc, copper, molybdenum, and nickel. Iron is the worst but it is a problem with all of them unless you have acidic water with zero KH. But few people have water with zero KH. All other nutrients are generally soluble.
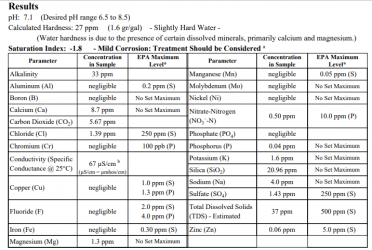
Typically tap water has close to zero iron, manganese. Zinc and Capper are generally present due to metal pipes. old iron pipes were often coated with zinc to preserve the iron. So in the past tap water ty[ically did have moree than enough zinc. However today most homes are made with copper pipe so copper is generally more than enough. However some new homes today often have plastic pipes. And some utilities are gradually replacing metal pipe in the ground with plastic.
Many people also state you want zero to 0.001ppm ofcopper if you have shrimp. However that is not true and shrimp and plants do need copper to live. In my tank I had breading shrimp I found a double dose of my micro fertilizer 0.020ppm of copper didn't have any effect on my shrimp. I don't use my tap water (too hard) but did have my copper measured and it was 0.050ppm of copper in it.
Molybdenum and nickel are only needed at about 0.001ppm for plants. Although I don't have any data I suspect these are commonly pressent at sufficient levels levels in most tanks.
IN larger tanks with a substantial fish load the the w waist can produce enough nutrients t to feed the plants with only an occasional water change instead of weekly water change. Some people can go months without a water change. In smaller tanks this can be difficult to achieve. In my 5 gallon shrimp tank a fertilizer is the only way I have to get enough nutrients.
One thing I can't figure out is why my pH is constantly at 7.8. It comes out of the tap at 6, if left out overnight to air out it will go up to 6.7, but after being in the tank it's always 7.8. I really don't know what is in the tank that's causing it to go so high.
Tap water typically has calcium and magnesium carbonate in it. After bing filtered at the the utility some chlorine is typically injected into it just before it goes into the water pipes. This chlorine reacts with some of the carbonate releasing CO2 in the water. So when water comes out of the tap it will have have some CO2. which will outgas. That can result in the PH going up and is likely happening. However keep in mind that CO2 is a week acid so other chemical reactions once the water comes out o the pipe might be occurring and affecting PH.
Also as plants consume nutrients in the water the PH in the tank can change. It is not unusual to to see a low PH in the morning with the lights off. But when the lights turn back on the plants can change the chemistry pushing it up or down in some case.. So just before the lights turn off the PH cold be substantially different. When I first saw this my pH In the morning was 7 but at the end of the day was 9. Dimming the light solved the problem keeping it close to 7. In this case plant need a lot of carbon and nitrogen, Plants can extract carbonate from the water leaving calcium and magneisum hydroxide. which push the PH up. In fertilizers potassium nitrate is often used as a source of potassium and nitrogen, Plants need a lot more nitrogen than potassium which can result in potassium hydroxide which again pushes the PH up. then when the lights turn off the hydroxides convert to carbonates and the pH drops.
In some tanks the PH drops a little bitt each day. this can be caused by GH boosters which have calcium / magnesium sulfate / chloride. Plants need more calcium and magnesium than sulfur and chloride. As a result plant growth chan result in excess acidity in the water until a water change. This problem can be solved by putting a decorative sea shell or crushed coral in the tank. The are mostly solid calcium carbonate. This will dissolve and neutralize acids pushing the PH to 7. In my tank one sea shell will dissolve in about a year.
However if the water goes acidic it won't up during the night . it will drop a little each day. I solved this problem with a decorative sea shell in the tank. The shell is solid KH and it will dissolve and neutralize acids in the water.



 /www.nilocg.com/shop/iron-chelate-11-dtpa-for-aquarium-plants/
/www.nilocg.com/shop/iron-chelate-11-dtpa-for-aquarium-plants/ There's so much to learn and watch out for. I'm trying to absorb it all.
There's so much to learn and watch out for. I'm trying to absorb it all.