Hello, I have been keeping fish on and off for the past 50 years. Mainly fresh water. This is my first forum of any type ever joined, old not that computer intelligent so please bear with me. Can you please answer a question on PH. My PH coming out of the tap is 9.4 according to my PH meter and aprox. with my liquid reagent tester. When I agitate a gallon of tap water in bucket for 24 hours my PH drops to 7.5 using my meter and approx. using my reagent tester. My understanding is if you agitate the co2 out of the water the PH will increase. Any help with this will be greatly appreicated.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
New Member
- Thread starter ricospook
- Start date
ChasingFish
Fish Fanatic
I don't personally know the answer, but I found this on the web...hopefully that helps:Hello, I have been keeping fish on and off for the past 50 years. Mainly fresh water. This is my first forum of any type ever joined, old not that computer intelligent so please bear with me. Can you please answer a question on PH. My PH coming out of the tap is 9.4 according to my PH meter and aprox. with my liquid reagent tester. When I agitate a gallon of tap water in bucket for 24 hours my PH drops to 7.5 using my meter and approx. using my reagent tester. My understanding is if you agitate the co2 out of the water the PH will increase. Any help with this will be greatly appreicated.
Attachments
ChasingFish
Fish Fanatic
This is my first forum of any type ever joined, old not that computer intelligent so please bear with me. Can you please answer a question on PH. My PH coming out of the tap is 9.4 according to my PH meter and aprox. with my liquid reagent tester. When I agitate a gallon of tap water in bucket for 24 hours my PH drops to 7.5 using my meter and approx. using my reagent tester. My understanding is if you agitate the co2 out of the water the PH will increase. Any help with this will be greatly appreicated.
Welcome to TFF.

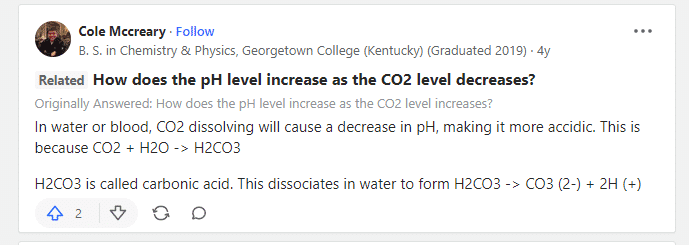
You are correct that the presence of dissolved CO2 in tap water causes the pH to lower due to the CO2 creating carbonic acid. Out-gassing the CO2 would in such situations raise the pH closer to its true level.
When the opposite occurs as here, it is probable that the water authority is adding something to increase the pH. Usually this is done in areas with naturally acidic water, but who knows. I would check their website to see if any substances are being added, for any reason. Also, what pH value do they indicate, just for comparison?
Uberhoust
Fish Herder
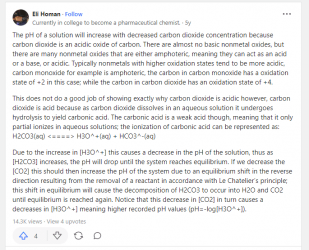
Just curious, I am having a hard time thinking what would lower the pH that quickly. CO2 absorption doesn't seem likely at normal CO2 concentrations (Actually checked before sending this and if you have low alkalinity the pH rate of change from atmospheric CO2 might be possible). I know they sometimes add sodium hydroxide to city water to protect the pipes from corrosion, is this material precipitating out. When you look at the bottom of the bucket after the pH lowers is there a fine white residue anywhere.
When the water comes out of the tap is it warmer or colder than when you take the pH reading. The CO2/Alkaline/Carbonic Acid relationship is very temperature dependent. Colder water can absorb more CO2.
The water will have to absorb CO2 to lower the pH and there isn't much normally in the air.
When the water comes out of the tap is it warmer or colder than when you take the pH reading. The CO2/Alkaline/Carbonic Acid relationship is very temperature dependent. Colder water can absorb more CO2.
The water will have to absorb CO2 to lower the pH and there isn't much normally in the air.
Hello , and thank you for your quick response. You asked if there was a fine white residue in the bucket and to my surprise there is. I use a food grade 5 gallon bucket with a small submersible pump to agitate the water and a heater to bring up the temperature. The color of the bucket is white and that’s why I didn’t notice it. I used a flashlight and could see small white particles floating around while being agitated, lots of them. I use API tap water conditioner which is just a dechlorinator. The dosage is 3 drops per gallon if you have chloramines which I do have, but I have heard in the summer water treatment plants increase the chloramines because of higher water temperatures, so I have been tripling the dose. Do you think that has anything to do with it? I know when that stuff dries on a surface it looks like small white flakes. For some reason I didn’t notice them when I added the water to my aquarium. I have only started agitating my water 24 hours for my partial water changes for the past 3 months, normally I would just draw the water out of the tap at the temperature I need, add my dechlorinator and agitate it for a few minutes. Now I use only the cold water and add the heater 1 or 2 hours before I use the water. I do like the fact that the PH is lower because I have 6 Green Neons and a Betta in a 5 gallon tank on my desk for about a year. I have owned tanks of all different sizes in the past 50 years from 10 to 125 gallons and I’m still learning new things all the time. I know that’s pushing it a little with that much fish for 5 gallons and I should know better. I’m surprised that the neon’s have lasted that long with my tap water parameters. I always use distilled water to replace evaporated water. I still would like to know why the PH is dropping in the bucket of agitated tap water when everything I read tells me the opposite. You said it might be sodium hydroxideI so I attached my water department water quality report for 2022, maybe you can see something in there that might help solve this. Thank you for your help.Just curious, I am having a hard time thinking what would lower the pH that quickly. CO2 absorption doesn't seem likely at normal CO2 concentrations (Actually checked before sending this and if you have low alkalinity the pH rate of change from atmospheric CO2 might be possible). I know they sometimes add sodium hydroxide to city water to protect the pipes from corrosion, is this material precipitating out. When you look at the bottom of the bucket after the pH lowers is there a fine white residue anywhere.
When the water comes out of the tap is it warmer or colder than when you take the pH reading. The CO2/Alkaline/Carbonic Acid relationship is very temperature dependent. Colder water can absorb more CO2.
The water will have to absorb CO2 to lower the pH and there isn't much normally in the air.
Attachments
Uberhoust
Fish Herder
First off, I am not a chemist, so take anything I say with a large grain of salt. I reviewed the treatment document, the one thing I noted is that lime is added to the water, then they re-acidify the water with CO2 early in the treatment process. I suspect that the water when it comes out of the tap it is not at equilibrium (for example my tap water can sit for days without the pH changing), in particular the water has too much lime and perhaps too much CO2.
I suspect when the water is at normal temperature and pressure it degasses some of the CO2 from the water causing the added lime to precipitate out. Under these conditions the pH of the water will be controlled by the relationship of the lime, CO2, and temperature of the water. But with material leaving the water, both the lime and C02, the pH changes overtime. The precipitated lime would be the white dust in the buckets. I am not entirely clear what would happen to the pH when both CO2 and the Lime leave the water at the same time.
What this means to you is that it is probably best to age your water to allow for the pH, GH, and KH to stabilize before using it for your fish. Additionally, you will still have to treat the water for Chloramine because that has been added, chloramines do not de-gas out of the water like straight chlorine can. Another interesting point is you have Floride added to your water, I honestly don't know what the implications for that are, both in its effect on the fish or on the water chemistry.
I suspect when the water is at normal temperature and pressure it degasses some of the CO2 from the water causing the added lime to precipitate out. Under these conditions the pH of the water will be controlled by the relationship of the lime, CO2, and temperature of the water. But with material leaving the water, both the lime and C02, the pH changes overtime. The precipitated lime would be the white dust in the buckets. I am not entirely clear what would happen to the pH when both CO2 and the Lime leave the water at the same time.
What this means to you is that it is probably best to age your water to allow for the pH, GH, and KH to stabilize before using it for your fish. Additionally, you will still have to treat the water for Chloramine because that has been added, chloramines do not de-gas out of the water like straight chlorine can. Another interesting point is you have Floride added to your water, I honestly don't know what the implications for that are, both in its effect on the fish or on the water chemistry.
Does your water provider add anything to raise pH? In areas with very soft acidic water, it is common for water providers to raise the pH of mains water to prevent pipe corrosion. The added chemicals often gas out on standing, or agitation of the water. But we also have members who live where the pH is artificially raised and the chemical doesn't gas out leaving the pH quite high for soft water.
Uberhoust
Fish Herder
They add both lime and CO2 to the water according to the water treatment document.Does your water provider add anything to raise pH?
Ah, I missed that 

Uberhoust
Fish Herder
I have just merged two threads so some of the posts seem out of order.
I have to go and cook dinner right now, I'll reply properly later.
I have to go and cook dinner right now, I'll reply properly later.
It's a long time since I studied chemistry and I've forgotten so much (I am 69 so university feels like a long time ago!). Your summary seems pretty accurate to me. I also suggest leaving new water to stand for a few days before using as this will allow things to stabilise before the water is added to the tank. The last thing you need is the gassing out/precipitation occurring in the tank. Possibly monitor a bucket of water daily to see how long it takes to stabilise, measuring pH, GH and KH if possible.
I want to say thank you for everyone’s help. I will definitely continue to agitate my water for at least 2 to 3 days prior to using it. No telling what I was subjecting my fish to by agitating the tap water only for a few minutes after adding my water conditioner. Fortunately I don’t change more than 25% at a time. Thanks Again.
Welcome to the forum... 
It seems that you've already collected a number of advices overhere regarding your problem.

It seems that you've already collected a number of advices overhere regarding your problem.
Similar threads
- Replies
- 10
- Views
- 250
- Replies
- 15
- Views
- 662
Most reactions
-
 397
397 -
 260
260 -
 197
197 -
 178
178 -
 177
177 -
 171
171 -
 168
168 -
 161
161 -
 146
146 -
 108
108 -
 106
106 -
 106
106 -
 99
99 -
 97
97 -
F
69