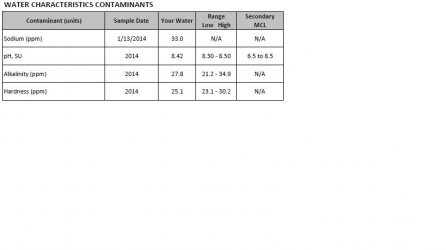
After I posted earlier today, it occurred to me that I had neglected to respond to your comment in post #1 about using chemicals to lower pH. I realize you said you don't want to, but I thought it might help if I explain why this is extremely risky to begin with. And it has largely to do with the GH and KH. The latter buffers the pH, preventing fluctuations. The higher the KH, the stronger this buffering will be. So if for example your source water happened to be moderately hard in terms of GH with a corresponding KH, any chemicals used to lower the pH without first dealing with lowering the GH/KH would send the pH down immediately, but the KH would kick in and the pH would rise back, usually in less than 24 hours. If you continue to add acids/chemicals to lower the pH, it will keep bouncing back, at least until the point at which you exhaust the buffering capacity, and then the pH could very suddenly crash as it is termed. All of this is severe on fish. This is why the only really safe way to lower pH is by diluting the source water to lower the GH and especially KH first.
And this brings me to your comment in post #12 that I obviously totally missed previously...my apology for that. But now I will comment on this.
While wood, along with peat, dried leaves, and similar organic matter will tend to lower the pH, this is kept in check by the KH as I've explained above. Only after the GH/KH is lowered will there be significant lowering of the pH from organics. And here I should mention that it is perfectly natural in every aquarium for the biological processes to slowly lower the pH. The production of CO2 is what achieves this; CO2 produces carbonic acid, increasing the acidity, thus lowering the pH. The breakdown of organics (fish excrement, etc) by bacteria in the substrate is primary in this, but other factors play into it as well. So if the GH/KH is sufficiently low in the source water, the pH will naturally lower in any aquarium.
In addition to the source water GH/KH, there are other ways to prevent this. Using calcareous substances as the gravel/sand substrate, or placing some of this in the filter, adding calcareous rock...each of these will slowly dissolve the "hard" minerals, primarily calcium and magnesium, thus raising GH, KH and pH. I would suspect this is occurring in the 29g. RO water is devoid of anything, so using this solely as the aquarium water means that the water parameters will be significantly affected by all sorts of things. And while only natural wood, peat or leaves in this case would certainly lower the pH and by quite a lot, there would appear here to be some calcareous substance doing the opposite. The composition of the substrate, or any such material in the filter media, are likely sources. RO water on its own should never be used in an aquarium; mixing it with some tap is better. And in your case, as it would appear the GH/KH may not be very high in the tap water [more on this below], this would probably be a good idea as it would work to stabilize things more. However, I would first want to ascertain the issue causing the rise in pH (and likely GH/KH) in this tank.
The 55g using tap water is lowering in pH, which suggests that the initial GH/KH of your tap water is likely not very high. If you are able to track this down on your water supplier's site, or directly, we will have confirmation (or not).
Water changes are the most significant impact on all of this, assuming the tanks are not overstocked to begin with, or over-fed; both of these will affect water chemistry a lot. So I would recommend increasing the volume of the weekly water changes to no less than 1/3 of the tank volume, and preferably more. I have been doing 50-60% changes every week for years, and I know this has helped me create an extremely stable water chemistry. My tap water is near-zero GH/KH, with a pH around 7, but my tanks run anywhere from 5 in some to mid-6 in others, depending upon the specifics of each. But these each remain absolutely constant; there is a change of no more than two or three decimal points with these water changes, indicating that the established biological system in each aquarium is fairly solid. However, if I neglected these water changes (which I never have, nor would), I would expect serious consequences, as the increased organics would overload the system rapidly.
Byron.
And this brings me to your comment in post #12 that I obviously totally missed previously...my apology for that. But now I will comment on this.
Here is the interesting thing I have yet to figure out... In my tank setups as far as what water I use, the 29gal uses RO water from the local fish room with a pH of 6.4 and yet the pH stays around 7.4 with natural driftwood in the 29gal. The 55gal uses my tap water with stays at 7.6 and yet the water in the aquarium after a day and a couple days after that stays around 6.7 with no natural driftwood. Water changes are done weekly on all my setups around 15-20%. Both tanks have always stayed the same... I use the API liquid testers and not the strips as I have realized in the beginning stages of my journey with fish keeping and the free testing done at the local petsmart is not reliable at all.
I guess that was part of why I started to lean towards SA cichlids on top of what I found out and in fear of going for African cichlids with using my tap water and it falling below certain parameters that would be unhealthy for them.
While wood, along with peat, dried leaves, and similar organic matter will tend to lower the pH, this is kept in check by the KH as I've explained above. Only after the GH/KH is lowered will there be significant lowering of the pH from organics. And here I should mention that it is perfectly natural in every aquarium for the biological processes to slowly lower the pH. The production of CO2 is what achieves this; CO2 produces carbonic acid, increasing the acidity, thus lowering the pH. The breakdown of organics (fish excrement, etc) by bacteria in the substrate is primary in this, but other factors play into it as well. So if the GH/KH is sufficiently low in the source water, the pH will naturally lower in any aquarium.
In addition to the source water GH/KH, there are other ways to prevent this. Using calcareous substances as the gravel/sand substrate, or placing some of this in the filter, adding calcareous rock...each of these will slowly dissolve the "hard" minerals, primarily calcium and magnesium, thus raising GH, KH and pH. I would suspect this is occurring in the 29g. RO water is devoid of anything, so using this solely as the aquarium water means that the water parameters will be significantly affected by all sorts of things. And while only natural wood, peat or leaves in this case would certainly lower the pH and by quite a lot, there would appear here to be some calcareous substance doing the opposite. The composition of the substrate, or any such material in the filter media, are likely sources. RO water on its own should never be used in an aquarium; mixing it with some tap is better. And in your case, as it would appear the GH/KH may not be very high in the tap water [more on this below], this would probably be a good idea as it would work to stabilize things more. However, I would first want to ascertain the issue causing the rise in pH (and likely GH/KH) in this tank.
The 55g using tap water is lowering in pH, which suggests that the initial GH/KH of your tap water is likely not very high. If you are able to track this down on your water supplier's site, or directly, we will have confirmation (or not).
Water changes are the most significant impact on all of this, assuming the tanks are not overstocked to begin with, or over-fed; both of these will affect water chemistry a lot. So I would recommend increasing the volume of the weekly water changes to no less than 1/3 of the tank volume, and preferably more. I have been doing 50-60% changes every week for years, and I know this has helped me create an extremely stable water chemistry. My tap water is near-zero GH/KH, with a pH around 7, but my tanks run anywhere from 5 in some to mid-6 in others, depending upon the specifics of each. But these each remain absolutely constant; there is a change of no more than two or three decimal points with these water changes, indicating that the established biological system in each aquarium is fairly solid. However, if I neglected these water changes (which I never have, nor would), I would expect serious consequences, as the increased organics would overload the system rapidly.
Byron.