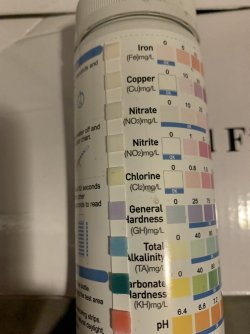
1st off... a big thank you to all those who have responded to my water chemistry questions... I'm really not that dense... but I do like to fully understand the what's? & whys?... I think I finally have gleaned enough information to understand that... 1) I should not be using whole house softened water for my tanks... 2) because of my water particular conditions, I need to install an RO unit for my aquarium water
I see happy fishes in my future... & to cheat, I've made up a spread sheet with "ideal" water parameters to strive for, for particular fish, & what the different scales equal, since there are often more that one way to record the readings...
I do have one more question that I haven't really found out... it concerns Alkalinity & Carbonate hardness... heard they are treated the same, as far as aquariums go, & that's why the numbers are the same for each reading on my test strips... but is there any way of changing one reading, without changing the other... in other words, for example, is there something, ( that is safe for the fish ) that could be used to increase carbonate hardness, that wouldn't increase the Alkalinity also??? I'm assuming it's actually the Carbonate hardness that acts as the buffer for our pH, & the Alkalinity is the result of raising or lowering the carbonate hardness, & the the Alkalinity in it's self, doesn't actually buffer the pH??? did I explain the question OK???
I see happy fishes in my future... & to cheat, I've made up a spread sheet with "ideal" water parameters to strive for, for particular fish, & what the different scales equal, since there are often more that one way to record the readings...
I do have one more question that I haven't really found out... it concerns Alkalinity & Carbonate hardness... heard they are treated the same, as far as aquariums go, & that's why the numbers are the same for each reading on my test strips... but is there any way of changing one reading, without changing the other... in other words, for example, is there something, ( that is safe for the fish ) that could be used to increase carbonate hardness, that wouldn't increase the Alkalinity also??? I'm assuming it's actually the Carbonate hardness that acts as the buffer for our pH, & the Alkalinity is the result of raising or lowering the carbonate hardness, & the the Alkalinity in it's self, doesn't actually buffer the pH??? did I explain the question OK???