-
Guest, the Tank of the Year contest is underway!
💧 Which tank will win? 👉 View the entries and vote now!!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ask Questions About Cycling
TwoTankAmin
Fish Maniac
Hi all- let me start with an apology to anybody who has read the piece and to tcamos whom I dove nuts re pictures and tables. In the final section on Suggestions and Trouble Shooting there is a table showing how much water to change. I had a heck of a fight getting it proper and it finally is. This is how it should look:
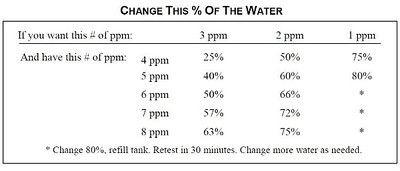
Tcamos the url for this is http /twotankamin.smugmug.com/photos/i-5xv9dJc/0/S/awing%20and%20a%20prayer%20small-S.jpg
/twotankamin.smugmug.com/photos/i-5xv9dJc/0/S/awing%20and%20a%20prayer%20small-S.jpg
I will also confess that I am basically net illiterate. This article looks beautiful as a Word document or even as a pdf, but posting it online trashes it. It should look much more pleasing. So if anybody out there is good with this side of things and wants to help make the final version looks its best on the site, please let me know.
Finally, I am more than happy to answer any questions anybody may have about why things were designed as they were, or how the numbers were calculated etc. please ask. If I did not state something clearly, it should be changed. If the math is off, it should be corrected.
On the non-technical side. If things are confusing in how they are said, please let me know and tell me how they can be improved. If they are too complicated for the newest of fish keepers, say so. One of the main goals of the piece was to make it easy to understand and then to use as a road map to get one's first tank cycled. This piece is written for only one reason- to get the person cycling their first tank to succeed in a reasonable time frame and with the commonly available/used tools. It is meant to reduce the number of posts seeking help for stalled or endless cycles from 1st timers.
Finally, if, after editing for changes suggested, folks feel this is a reasonably helpful piece for first time cyclers, I am prepared to do a few more starting with test kits and then on more advanced cycling with an emphasis on seeding and cycling with plants.

Tcamos the url for this is http
 /twotankamin.smugmug.com/photos/i-5xv9dJc/0/S/awing%20and%20a%20prayer%20small-S.jpg
/twotankamin.smugmug.com/photos/i-5xv9dJc/0/S/awing%20and%20a%20prayer%20small-S.jpgI will also confess that I am basically net illiterate. This article looks beautiful as a Word document or even as a pdf, but posting it online trashes it. It should look much more pleasing. So if anybody out there is good with this side of things and wants to help make the final version looks its best on the site, please let me know.
Finally, I am more than happy to answer any questions anybody may have about why things were designed as they were, or how the numbers were calculated etc. please ask. If I did not state something clearly, it should be changed. If the math is off, it should be corrected.
On the non-technical side. If things are confusing in how they are said, please let me know and tell me how they can be improved. If they are too complicated for the newest of fish keepers, say so. One of the main goals of the piece was to make it easy to understand and then to use as a road map to get one's first tank cycled. This piece is written for only one reason- to get the person cycling their first tank to succeed in a reasonable time frame and with the commonly available/used tools. It is meant to reduce the number of posts seeking help for stalled or endless cycles from 1st timers.
Finally, if, after editing for changes suggested, folks feel this is a reasonably helpful piece for first time cyclers, I am prepared to do a few more starting with test kits and then on more advanced cycling with an emphasis on seeding and cycling with plants.
Blondielovesfish
Kia Ora
Just curious TTA, is a thread about fish in cycling going to be written or are we going to continue using the old thread?
A very useful article and well-written!
Can I just ask for clarification on a couple of things?
Can I just ask for clarification on a couple of things?
Presumably you are saying both conditions have to be met before adding the 2nd dose of ammonia. So if ammonia is under 0.75ppm but nitrIte isn't over 2ppm - don't add any more ammonia. Or is it that when ammonia gets to less than 0.75ppm we should be seeing nitrItes over 2ppm?
If at any time you test and ammonia is under .75 ppm and nitrite is clearly over 2 ppm, it is time to add more ammonia. Add the same full amount as you did the first time.
I will shortly be getting some Dr Tim's One and Only - should I follow the above routine or should it be different?If you can add gravel or filter media from a healthy cycled tank, it will accelerate the process. The more you can add, the more it will help. If you can do this, reduce the time between testing from every 3 days to every 2 days. Test on days 3, 5, 7, 9 etc. Then reduce the every 2 day testing to every day.
TwoTankAmin said:Hi all- let me start with an apology to anybody who has read the piece and to tcamos whom I dove nuts re pictures and tables. In the final section on Suggestions and Trouble Shooting there is a table showing how much water to change. I had a heck of a fight getting it proper and it finally is. This is how it should look:

I've updated the thread to include this graphic.
BTW, I haven't had a chance to tell you TwoTank... Great job! Thank you!
I will shortly be getting some Dr Tim's One and Only - should I follow the above routine or should it be different?Mamashack said:A very useful article and well-written!
Can I just ask for clarification on a couple of things?
Presumably you are saying both conditions have to be met before adding the 2nd dose of ammonia. So if ammonia is under 0.75ppm but nitrIte isn't over 2ppm - don't add any more ammonia. Or is it that when ammonia gets to less than 0.75ppm we should be seeing nitrItes over 2ppm?
If at any time you test and ammonia is under .75 ppm and nitrite is clearly over 2 ppm, it is time to add more ammonia. Add the same full amount as you did the first time.
If you can add gravel or filter media from a healthy cycled tank, it will accelerate the process. The more you can add, the more it will help. If you can do this, reduce the time between testing from every 3 days to every 2 days. Test on days 3, 5, 7, 9 etc. Then reduce the every 2 day testing to every day.
I'd follow the directions that Dr. Tim gives for his product, to the letter.
TwoTankAmin
Fish Maniac
Mama- I am saying both conditions should be met. It is sort of a double check process. The nitrite kits are pretty hard to read at times. By asking folks to be sure they clearly have nitrites it becomes more difficult to make a mistake in testing ammonia. One needs to be sure they are converting the ammonia into nitrite. Plus by wanting 2 ppm or higher, we know nitrites are going up.
Your question regarding Dr. Tim's gives me an opportunity to explain why there is a difference between his instructions, which you should definitely follow, and mine. You will see both methods start out similarly except we suggest a lower limit on ammonia be used. When he says don't exceed 5 ppm Ammonia-n, that is about 6.5 ppm om an API kit. We are using 3 ppm which is less than half. This is why at the end I tell folks "fully stock an average amount of fish for one's tank size and not to stock heavily or to overstock." A more experienced fish keeper who can seed a tank, who is more likely to have live plants, is a more proficient tester can do it differently.
But it is the next step where what I wrote and what Dr. H. suggests differ. He adds the next ammonia dose as soon as nitrites appear which is about day 5. I have suggested one wait to see nitrites clearly rising (2ppm or more) to add a 2nd full dose. There is a reason for the difference in the two methods which becomes apparent when you compare what happens next.
Dr. Hovanec instructs one to begin adding a 1/4 dose of ammonia every few days making sure nitrite does not exceed 5 ppm Nitrite-n. And here is where it gets too complicated for the 1st time cycler. That 5 pppm is going to show up on an API kits as about 16.5 ppm. But that kit uses the total ion scale not the -n scale, so the kit needs to read 16.5 ppm to let you know the Nitrite-n is at that 5 ppm line.
But the API kit stops at 5 ppm. This means one needs to be performing diluted tests. These should not be done with tap water because it is almost certain to contain things that will affect the test results. One should be using ro/di or distilled water for this. And because Dr. H wants one to track a moving target multiple tests like this need to be performed. I do not think that a newbie trying to get their first tank cycled in an easy simple way needed to have a method they could manage.
And if you compare both methods and assume in Dr. Hovanec's one will likely do about 3 ammonia 1/4 doses he will have one adding 3ppm + 1.5ppm + 2.25 = 6.75 before the final 24 hours test. The method I suggest has you add 3ppm + 3ppm + 1ppm =7.0 ppm. What differs is the timing. The method I have devised should never result in nitrite getting high enough to mess up the cycle without having to do diluted nitrite testing.
NH3 = NH3-N * 1.21589 (1 ppm on an API kit = .83 ppm-n)
NH4 = NH4-N * 1.28786 (1 ppm on an API kit = .78 ppm-n)
NO2 = NO2-N * 3.28443 (1 ppm on an API kit = .30 ppm-n)
NO3 = NO3-N * 4.42664 (1 ppm on an API kit = .23 ppm-n)
Most of us should still recall what things were like when we cycled our first tank. All of this stuff is a whole lot more confusing. Making mistakes is easy. My goal was to try and eliminate the mistakes by making the method less complicated and very unlikely to fail.
Mama if you follow his instructions to the letter, and the bottle has not been mishandled along the way to harm the bacteria, you should be able to cycle your new tank in a week (realistic time frame 5-9 days). I hope you also got his ammonium chloride solution. My seeding note was not for one using a bottled bacteria at full strength where you know how much seeding you are doing. They are for folks who have some seeding to help.
Here are his directions:
 /www.drtimsaquatics.com/resources/how-to-start
/www.drtimsaquatics.com/resources/how-to-start
Incidentally that page now has a new chart illustrating a fishless cycle using his method. It is scaled in -N. Accurate as all get out and likely confusing to the 1st timer (and others I bet). He is ever the consummate scientist and has got it all perfectly correct. Its just going to confuse the heck out of the 1st time cycler, imo.
Blondie- I would love to do an article on that. It really is a 2 part one since there is how to cycle with fish correctly from the start, which is not the preferred method. And then there are those who without a good understanding who just start one and hit the wall. One is a how to and the other a rescue. Read my last post in this thread for clarification http /www.fishforums.net/index.php?/topic/421090-cycling-section/page-3#entry3557681 I think we can mostly convince people to go fishless when they have not yet begun the cycle, its the rescue folks who need help.
/www.fishforums.net/index.php?/topic/421090-cycling-section/page-3#entry3557681 I think we can mostly convince people to go fishless when they have not yet begun the cycle, its the rescue folks who need help.
Your question regarding Dr. Tim's gives me an opportunity to explain why there is a difference between his instructions, which you should definitely follow, and mine. You will see both methods start out similarly except we suggest a lower limit on ammonia be used. When he says don't exceed 5 ppm Ammonia-n, that is about 6.5 ppm om an API kit. We are using 3 ppm which is less than half. This is why at the end I tell folks "fully stock an average amount of fish for one's tank size and not to stock heavily or to overstock." A more experienced fish keeper who can seed a tank, who is more likely to have live plants, is a more proficient tester can do it differently.
But it is the next step where what I wrote and what Dr. H. suggests differ. He adds the next ammonia dose as soon as nitrites appear which is about day 5. I have suggested one wait to see nitrites clearly rising (2ppm or more) to add a 2nd full dose. There is a reason for the difference in the two methods which becomes apparent when you compare what happens next.
Dr. Hovanec instructs one to begin adding a 1/4 dose of ammonia every few days making sure nitrite does not exceed 5 ppm Nitrite-n. And here is where it gets too complicated for the 1st time cycler. That 5 pppm is going to show up on an API kits as about 16.5 ppm. But that kit uses the total ion scale not the -n scale, so the kit needs to read 16.5 ppm to let you know the Nitrite-n is at that 5 ppm line.
But the API kit stops at 5 ppm. This means one needs to be performing diluted tests. These should not be done with tap water because it is almost certain to contain things that will affect the test results. One should be using ro/di or distilled water for this. And because Dr. H wants one to track a moving target multiple tests like this need to be performed. I do not think that a newbie trying to get their first tank cycled in an easy simple way needed to have a method they could manage.
And if you compare both methods and assume in Dr. Hovanec's one will likely do about 3 ammonia 1/4 doses he will have one adding 3ppm + 1.5ppm + 2.25 = 6.75 before the final 24 hours test. The method I suggest has you add 3ppm + 3ppm + 1ppm =7.0 ppm. What differs is the timing. The method I have devised should never result in nitrite getting high enough to mess up the cycle without having to do diluted nitrite testing.
NH3 = NH3-N * 1.21589 (1 ppm on an API kit = .83 ppm-n)
NH4 = NH4-N * 1.28786 (1 ppm on an API kit = .78 ppm-n)
NO2 = NO2-N * 3.28443 (1 ppm on an API kit = .30 ppm-n)
NO3 = NO3-N * 4.42664 (1 ppm on an API kit = .23 ppm-n)
Most of us should still recall what things were like when we cycled our first tank. All of this stuff is a whole lot more confusing. Making mistakes is easy. My goal was to try and eliminate the mistakes by making the method less complicated and very unlikely to fail.
Mama if you follow his instructions to the letter, and the bottle has not been mishandled along the way to harm the bacteria, you should be able to cycle your new tank in a week (realistic time frame 5-9 days). I hope you also got his ammonium chloride solution. My seeding note was not for one using a bottled bacteria at full strength where you know how much seeding you are doing. They are for folks who have some seeding to help.
Here are his directions:
Full text here http
Using DrTim’s Aquatics One & Only Live Bacteria: The best and easiest way to fishless cycle is to combine adding the ammonium chloride with our Live Nitrifying bacteria. When used in combination, these will cycle the tank in less than one week. Again, do not add too much ammonia. We make it easy by providing a bottle of reagent grade ammonium chloride that is at a concentration such that adding 1 drop of solution to 1 gallon of aquarium water will result in an ammonia-nitrogen concentration of 2 mg/L (ppm).
The procedure is to add the ammonium chloride solution, shake the bottle of nitrifying bacteria well and add it to the aquarium. Measure ammonia and nitrite the next day and record. Add ½ dose and wait 24 hours and measure again. By day 5 to 7, you should be able to add 1 drop per gallon and the next day, ammonia and nitrite will be 0.
 /www.drtimsaquatics.com/resources/how-to-start
/www.drtimsaquatics.com/resources/how-to-startIncidentally that page now has a new chart illustrating a fishless cycle using his method. It is scaled in -N. Accurate as all get out and likely confusing to the 1st timer (and others I bet). He is ever the consummate scientist and has got it all perfectly correct. Its just going to confuse the heck out of the 1st time cycler, imo.
Blondie- I would love to do an article on that. It really is a 2 part one since there is how to cycle with fish correctly from the start, which is not the preferred method. And then there are those who without a good understanding who just start one and hit the wall. One is a how to and the other a rescue. Read my last post in this thread for clarification http
 /www.fishforums.net/index.php?/topic/421090-cycling-section/page-3#entry3557681 I think we can mostly convince people to go fishless when they have not yet begun the cycle, its the rescue folks who need help.
/www.fishforums.net/index.php?/topic/421090-cycling-section/page-3#entry3557681 I think we can mostly convince people to go fishless when they have not yet begun the cycle, its the rescue folks who need help.I'm not sure what to do with the ammonia conversion part of your table above as my API test kit only does a combined NH3+NH4+ reading not individual readings as in your table so is there a combined conversion figure for that? I also have the Salifert ammonia test, but again that is the combined test reading rather than the individual readings.
I'm comfortable having a go with the conversion for the addition of ammonium chloride (I'll be using Kleenoff which is aquarium safe and the strength is known), but I'm not sure how I'd check the results using the combined tests (NH3+NH4+). Both the API and Salifert NitrIte test can be converted easily enough by multiplying the reading by 0.3 as you stated. I'm just puzzled how I'd convert the combined ammonia readings to -N. This would be particularly helpful if I am to follow Dr Tim's instructions to the letter.
I'm comfortable having a go with the conversion for the addition of ammonium chloride (I'll be using Kleenoff which is aquarium safe and the strength is known), but I'm not sure how I'd check the results using the combined tests (NH3+NH4+). Both the API and Salifert NitrIte test can be converted easily enough by multiplying the reading by 0.3 as you stated. I'm just puzzled how I'd convert the combined ammonia readings to -N. This would be particularly helpful if I am to follow Dr Tim's instructions to the letter.
NH3 = NH3-N * 1.21589 (1 ppm on an API kit = .83 ppm-n)
NH4 = NH4-N * 1.28786 (1 ppm on an API kit = .78 ppm-n)
NO2 = NO2-N * 3.28443 (1 ppm on an API kit = .30 ppm-n)
NO3 = NO3-N * 4.42664 (1 ppm on an API kit = .23 ppm-n)
Use the higher conversion value, 1.28786, since the goal is to keep it under a specific value. Using the higher level will keep you from going over that threshold. The specific numbers are less important, as long as you are under the given maximums, as I understand it.
Ok thanks eagles! I was wondering whether it would be as simple as working out the average of the 2 figures, but your explanation makes sense so that's what I'll do. Am making a spread-sheet using excel as I'll get confused otherwise. I've not worked in -N before so it will be an interesting experience!
Well, to be honest, if you wanted to be more accurate, you could determine the ratio of NH3 to NH4 based on the pH, and then you could do a weighted average, but personally, I'd say that's far too much effort for nearly no real benefit.
TwoTankAmin
Fish Maniac
Mama- you are not working in ammonia-n etc. You are still using your API kits. If you want to translate his numbers, use the chart I posted earlier.
NH3 and NH4 have slightly different weights due to the the extra H in NH4.
N= 14.007
H = 1.0079
NH3 = 17 and NH4 = 18 and that accounts for the difference. It is also why NH3 is more toxic. It is smaller and can fit where NH4 can't and that gets it inside the fish. the odd part is once inside it turns into NH4 because blood pH is higher inside the fish.
You will often find the ammonia conversion stated as 1.3 - supposedly rounded up from 1.28. In fact the exact weight would be a combination of the weights of NH3 present in the test and then the NH4. But the reality is it is never above a small number. I use the 1.28.
The key to understanding the API type test kits results is to look at the atomic weighs of all of it all, Nitrite is NO2
N= 14.007
O= 15.999
NO2 = 14.007 + 31.998 = 46.005
NO3 = 14.007 + 47.997 = 62.004
Note, there is one N at each stage- that lets scientists add up the nitrogen and ignore the rest. So they use the N scale and the scale of N is constant from start to finish.
The test kits we use count in the other stuff which means there is a magnification of the ppms each step.
1 ppm of ammonia becomes 46 /17.9 times as much nitrite or 2.6 times and that becomes 62/17.9 or 3.5 times.
3 ppm of ammonia becomes 7.8 ppm nitrite and 10.5 ppm of nitrate.
I have folks do two additions of 3 ppm.:
6 ppm of ammonia becomes 15.6 ppm of nitrite and 21 ppm of nitrate.
But the fact is the nitrite will start to turn into nitrate along the way and that will prevent the nitrite from getting up to that theoretical maximum. And by the time the 1/3 maint. addition goes in, nitrite is falling sharply and the addition wont really slow down that side of the cycle by much at all.
The numbers mean the amounts given in the directions and the time to do things are designed to make it impossible for the nitrite to reach the danger level on an API kit of 16.5 ppm of nitrite. Further, they are designed to prevent the accumulation of ammonia similarly. 5 ppm ammonia-n = 6.5 ppm on the test kit I have only 6 added in two spaced additions. The only problems hat might come in relation to low pH, KH and/or O.
However, there was only so much to put into the article, I mention these three things but that is all. I still need no to write a testing article to try and explain all of the above clearly. But also to show when and where kit results can go wrong and why. There is a reason for those pesky .25 ppm ammonia readings that are really not there. There is a reason nitrate kits can be unreliable, especially during a cycle when nitrite is present as well.
One of my hopes in doing some of these article was to create a place to send folks to read when those same old Qs come up. I would rather see the OP told to read here, then come back with your Qs than have a bunch of people all trying to explain parts and sometimes wrongly so. On average i read about 5-20 abstracts and peruse several full research papers on related topics every week and have now for a few years. And I am still a rank amateur. But I know a lot more than I used to and have disabused myself of a lot of urban aquarium myths along the way. I just want to share that with folks because the info will help one be a better informed fish keeper I believe and hope.
One last note mama- Dr T's directions may be a tad confusing. On day two you test and add the half dose. His directions will not let you overdose doing that. It doesn't matter what numbers you test at. If you start at one drop/gal you hit 3 ppm ammonia-n (2ppm + 1 ppm), if you start at 1.5 drops you hit 4.5 ppm (3 ppm + 1.5 ppm). Both are under the 5 ppm-n danger line. Remember, he works in the -nitrogen (-n) scale. so just do the day two 1/2 dose w/o question

NH3 and NH4 have slightly different weights due to the the extra H in NH4.
N= 14.007
H = 1.0079
NH3 = 17 and NH4 = 18 and that accounts for the difference. It is also why NH3 is more toxic. It is smaller and can fit where NH4 can't and that gets it inside the fish. the odd part is once inside it turns into NH4 because blood pH is higher inside the fish.
You will often find the ammonia conversion stated as 1.3 - supposedly rounded up from 1.28. In fact the exact weight would be a combination of the weights of NH3 present in the test and then the NH4. But the reality is it is never above a small number. I use the 1.28.
The key to understanding the API type test kits results is to look at the atomic weighs of all of it all, Nitrite is NO2
N= 14.007
O= 15.999
NO2 = 14.007 + 31.998 = 46.005
NO3 = 14.007 + 47.997 = 62.004
Note, there is one N at each stage- that lets scientists add up the nitrogen and ignore the rest. So they use the N scale and the scale of N is constant from start to finish.
The test kits we use count in the other stuff which means there is a magnification of the ppms each step.
1 ppm of ammonia becomes 46 /17.9 times as much nitrite or 2.6 times and that becomes 62/17.9 or 3.5 times.
3 ppm of ammonia becomes 7.8 ppm nitrite and 10.5 ppm of nitrate.
I have folks do two additions of 3 ppm.:
6 ppm of ammonia becomes 15.6 ppm of nitrite and 21 ppm of nitrate.
But the fact is the nitrite will start to turn into nitrate along the way and that will prevent the nitrite from getting up to that theoretical maximum. And by the time the 1/3 maint. addition goes in, nitrite is falling sharply and the addition wont really slow down that side of the cycle by much at all.
The numbers mean the amounts given in the directions and the time to do things are designed to make it impossible for the nitrite to reach the danger level on an API kit of 16.5 ppm of nitrite. Further, they are designed to prevent the accumulation of ammonia similarly. 5 ppm ammonia-n = 6.5 ppm on the test kit I have only 6 added in two spaced additions. The only problems hat might come in relation to low pH, KH and/or O.
However, there was only so much to put into the article, I mention these three things but that is all. I still need no to write a testing article to try and explain all of the above clearly. But also to show when and where kit results can go wrong and why. There is a reason for those pesky .25 ppm ammonia readings that are really not there. There is a reason nitrate kits can be unreliable, especially during a cycle when nitrite is present as well.
One of my hopes in doing some of these article was to create a place to send folks to read when those same old Qs come up. I would rather see the OP told to read here, then come back with your Qs than have a bunch of people all trying to explain parts and sometimes wrongly so. On average i read about 5-20 abstracts and peruse several full research papers on related topics every week and have now for a few years. And I am still a rank amateur. But I know a lot more than I used to and have disabused myself of a lot of urban aquarium myths along the way. I just want to share that with folks because the info will help one be a better informed fish keeper I believe and hope.
One last note mama- Dr T's directions may be a tad confusing. On day two you test and add the half dose. His directions will not let you overdose doing that. It doesn't matter what numbers you test at. If you start at one drop/gal you hit 3 ppm ammonia-n (2ppm + 1 ppm), if you start at 1.5 drops you hit 4.5 ppm (3 ppm + 1.5 ppm). Both are under the 5 ppm-n danger line. Remember, he works in the -nitrogen (-n) scale. so just do the day two 1/2 dose w/o question
Similar threads
- Replies
- 2
- Views
- 2K
- Replies
- 5
- Views
- 2K


