Miss Wiggle
Practically perfect in every way
Right, been wondering about ammonium a bit and got a few questions, just wanted to prompt a bit of debate to improve my understanding.
so products like ammo lock say that they deal with ammonia in the tank, we know what they do is convert it to ammonium which can still be used by the filter bacteria so the tank still cycles and it's less harmful to fish.
so questions
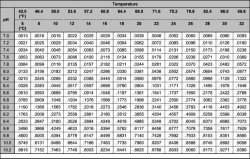
1 - is ammonium harmful to fish at all, is it just 'less toxic' to fish than ammonia or is it 'non-toxic'. If it is still toxic then at what concentrations to we need to be concerned?
2 - do the filter bacteria prefer ammonia or ammonium as a 'food' source, will they readily take either, if for example you were doing a fish-in cycle and using ammo-lock to control the ammonia is the cycle likely to take longer or anything like that. What effect does it have?
3 - what actually is ammonium, i assume it's some chemical compound made up of ammonia and something else, so what is it?
any other exciting and useful info on ammonium I'd love to hear it.
Thanks
so products like ammo lock say that they deal with ammonia in the tank, we know what they do is convert it to ammonium which can still be used by the filter bacteria so the tank still cycles and it's less harmful to fish.
so questions
1 - is ammonium harmful to fish at all, is it just 'less toxic' to fish than ammonia or is it 'non-toxic'. If it is still toxic then at what concentrations to we need to be concerned?
2 - do the filter bacteria prefer ammonia or ammonium as a 'food' source, will they readily take either, if for example you were doing a fish-in cycle and using ammo-lock to control the ammonia is the cycle likely to take longer or anything like that. What effect does it have?
3 - what actually is ammonium, i assume it's some chemical compound made up of ammonia and something else, so what is it?
any other exciting and useful info on ammonium I'd love to hear it.
Thanks




 /www.fishforums.net/index.php?showtopic=154313&
/www.fishforums.net/index.php?showtopic=154313&