Don't forget to ascertain the GH and KH of your source water. Those levels are part of the whole picture no matter what you end up doing. It is possible that the pH is the source water, and the GH and KH will help to pin this down.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Help - tank levels not as expected in fishless tank
- Thread starter CV26
- Start date
Our water company's website says our average PH should be 7.37 and we have moderately hard water.
It doesn't seem to give a GH and KH value although I'll admit I don't quite know what I'm looking for so will do some more research later.
Nobody in any of the shops has discussed water hardness with us. Can you buy conditioners to help with things like that?
It doesn't seem to give a GH and KH value although I'll admit I don't quite know what I'm looking for so will do some more research later.
Nobody in any of the shops has discussed water hardness with us. Can you buy conditioners to help with things like that?
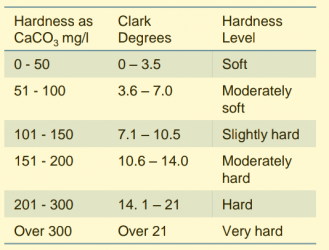
Does the water company give a number for hardness (and the unit) It is not unknown for a water company to use the term moderately hard when fishkeepers would call the level slightly hard. That's why numbers are more useful.
Changing hardness can be complex. To harden water you would add salts such as remineralisation salts. To soften water, it is mixed with reverse osmosis water (pure water) or rain water. But all water changes must be done with treated water, and salts/RO water work out expensive long term. It is much easier to work with whatever comes out of your tap, and keep fish that are happy in that water.
Changing hardness can be complex. To harden water you would add salts such as remineralisation salts. To soften water, it is mixed with reverse osmosis water (pure water) or rain water. But all water changes must be done with treated water, and salts/RO water work out expensive long term. It is much easier to work with whatever comes out of your tap, and keep fish that are happy in that water.
So I've only tested PH tonight as that was giving us our strongest jump yesterday. Figured we would test for ammonia and nitrites tomorrow after a bit more brew time.
Here are the results...
Tap water (no dechlorinator) in glass left open for 24 hours - now reads 8.0.
Water from tank after 75% water change and 24 hours. (In the tank is the gravel and the plants plus decholrinator. Heater and pump have been running.) - PH is 8.0
3 separate buckets each with dechlorinated tap water left for 24 hours and one item of either light rock, dark rock or bog wood. No heating or filter but our house temp has been a consistent 22 degrees all day. They all give a PH of 7.4
I also retested the jug of water with the gravel in it that will now have been standing for 48 hours. That came up as 7.4 too.
I'm confused!
I've retested everything and got the same results so confident there's no operator error at the moment.
If the standing tap water with nothing in it settles at 8.0, shouldn't everything else do that and we'd say oh well it's our water?
I don't understand why the tank is at 8.0 but the rest are coming up at 7.4 - there is a definite colour difference between the samples.
And why would the tank with the gravel read 8.0 but the jug with the gravel come in at 7.4?
I hope all that makes sense! Any insights?
Here are the results...
Tap water (no dechlorinator) in glass left open for 24 hours - now reads 8.0.
Water from tank after 75% water change and 24 hours. (In the tank is the gravel and the plants plus decholrinator. Heater and pump have been running.) - PH is 8.0
3 separate buckets each with dechlorinated tap water left for 24 hours and one item of either light rock, dark rock or bog wood. No heating or filter but our house temp has been a consistent 22 degrees all day. They all give a PH of 7.4
I also retested the jug of water with the gravel in it that will now have been standing for 48 hours. That came up as 7.4 too.
I'm confused!
I've retested everything and got the same results so confident there's no operator error at the moment.
If the standing tap water with nothing in it settles at 8.0, shouldn't everything else do that and we'd say oh well it's our water?
I don't understand why the tank is at 8.0 but the rest are coming up at 7.4 - there is a definite colour difference between the samples.
And why would the tank with the gravel read 8.0 but the jug with the gravel come in at 7.4?
I hope all that makes sense! Any insights?
Is it at all possible that, in smaller volumes of water like the jug and buckets, the rocks, gravel and bogwood could actually be lowering the PH of the surrounding water? Something that, in an 100l tank, they couldn't do effectively.
Just pondering...
Just pondering...
Looking just at your hardness, the number you want is Hardness German 9.82.
There are 2 units used in fishkeeping. dH is the same as German degrees, so your hardness in 9.82 dH. The other unit is ppm, and dH 9.82 converts to 176 ppm. Remember those two figures as fish profiles will use one or other unit.
I don't consider that to be moderately hard, more like slightly hard. There are quite a few soft water fish that would be happy in that, and quite a few hard water fish that would not like it at all as it's too soft for them.
There are 2 units used in fishkeeping. dH is the same as German degrees, so your hardness in 9.82 dH. The other unit is ppm, and dH 9.82 converts to 176 ppm. Remember those two figures as fish profiles will use one or other unit.
I don't consider that to be moderately hard, more like slightly hard. There are quite a few soft water fish that would be happy in that, and quite a few hard water fish that would not like it at all as it's too soft for them.
Essjay resolved your GH questions. I agree with her fish assessment too.
As for the variable pH mentioned in post #22, that is puzzling. Taken individually...I can understand the pH of 7.4 after 24 hours regardless of the rock/wood as this value basically matches the water authority data you cited. The pH of 8.0 coming up in the 24-hour pail of plain tap water or the aquarium is puzzling.
There is another possibility...the water authority may be adding something to increase pH. This is more common in very soft water areas (like I have) where an acidic pH can corrode pipes and such, so they add something (soda ash is one substance) to increase pH but this is temporary and in an aquarium dissipates out after a day or less. Might be worth checking your water site again to see just what they add to the water. Chlorine I would assume, but anything else?
It is possible that the source water is around 7.3 or 7.4, and something calcareous in the tank is raising it to 8. I know rocks and gravel have been separated out in buckets, but it might still be something taken together doing this. Maybe add a bit of each to as bucket, or two buckets, and see if they change? I mean, each type of rock and some gravel in two different buckets of plain tap water, for 24 hours.
As for the variable pH mentioned in post #22, that is puzzling. Taken individually...I can understand the pH of 7.4 after 24 hours regardless of the rock/wood as this value basically matches the water authority data you cited. The pH of 8.0 coming up in the 24-hour pail of plain tap water or the aquarium is puzzling.
There is another possibility...the water authority may be adding something to increase pH. This is more common in very soft water areas (like I have) where an acidic pH can corrode pipes and such, so they add something (soda ash is one substance) to increase pH but this is temporary and in an aquarium dissipates out after a day or less. Might be worth checking your water site again to see just what they add to the water. Chlorine I would assume, but anything else?
It is possible that the source water is around 7.3 or 7.4, and something calcareous in the tank is raising it to 8. I know rocks and gravel have been separated out in buckets, but it might still be something taken together doing this. Maybe add a bit of each to as bucket, or two buckets, and see if they change? I mean, each type of rock and some gravel in two different buckets of plain tap water, for 24 hours.
Thanks Byron. We have had weeks of very hot weather here in the UK so there's a good chance the water company have been doing more recycling of existing supplies. We've since had a glut of rain so I dont know whether that might have flushed extra minerals into the supply again which might result in a change.
The WC have recorded a high of 7.8 last year so a higher read is known for the area. We'll keep an eye on it and I'll mention your extra test ideas to my husband when he gets back home.
As for Ammomia and Nitrites, well we couldn't resist testing tonight. And we have a winner!
Bog wood - A 0 N maybe a 0.1, not strong enough for the full 0.25 but not quite the flat blue of 0
Light rock - A 0 N 0.5
Dark rock - A 0 N 0
Tank - A 0 N maybe a 0.1, not strong enough for the full 0.25 but not quite the flat blue of 0
That light rock looks like it has something growing on it and its very craggy so it could well be harbouring something. It wont be going back in the tank.
We'll keep everything separate for aother 24 hours and retest tomorrow before deciding to reconstruct the tank.
The WC have recorded a high of 7.8 last year so a higher read is known for the area. We'll keep an eye on it and I'll mention your extra test ideas to my husband when he gets back home.
As for Ammomia and Nitrites, well we couldn't resist testing tonight. And we have a winner!
Bog wood - A 0 N maybe a 0.1, not strong enough for the full 0.25 but not quite the flat blue of 0
Light rock - A 0 N 0.5
Dark rock - A 0 N 0
Tank - A 0 N maybe a 0.1, not strong enough for the full 0.25 but not quite the flat blue of 0
That light rock looks like it has something growing on it and its very craggy so it could well be harbouring something. It wont be going back in the tank.
We'll keep everything separate for aother 24 hours and retest tomorrow before deciding to reconstruct the tank.
ah you big pussies, 28C in the sun, bah. If it's not 40C in the shade, it's not hotThanks Byron. We have had weeks of very hot weather here in the UK so there's a good chance the water company have been doing more recycling of existing supplies. We've since had a glut of rain so I dont know whether that might have flushed extra minerals into the supply again which might result in a change.
On a serious note tho, if there has been lots of rain washing stuff into the water supply, the water corporation might be adding more chlorine/ chloramine to make sure nothing is alive. So make sure you use a good dechlorinator on any new water before it is added to the tank. They do the same thing when the weather warms up, and I mean warms up, not 28C
And no I am not going to let you guys think 28C is hot
Yep we Brits moan about it being cold but then we'll moan when it's hot too!  To be fair we did have a few 31 days in there...
To be fair we did have a few 31 days in there...
Thanks for all the help.
Game plan now is to retest tonight and if all looks ok we will redo the tank minus the light rock. Then we can retest in a few days and go from there.
Thanks for all the help.
Game plan now is to retest tonight and if all looks ok we will redo the tank minus the light rock. Then we can retest in a few days and go from there.
LOLYep we Brits moan about it being cold but then we'll moan when it's hot too!To be fair we did have a few 31 days in there...
Well the light rock bucket nitrites have shot up to 1.0 and there is maybe a 0.25 Ammonia reading too. Nitrates have stayed the same so no stage 2 bacteria involved.
Dark rock hasn't affected the water at all.
Bog wood and tank though both have maybe a small trace of Ammonia and 0.25 reading of Nitrites. This suggests that whatever was on the rock has moved into the tank and wood somewhat.
However we have also had a few of the plants shed some leaves. Not a lot but maybe a leaf every day or two. And the bogwood is organic itself so might be decaying a bit too.
Just wondering what to do now?
Dark rock hasn't affected the water at all.
Bog wood and tank though both have maybe a small trace of Ammonia and 0.25 reading of Nitrites. This suggests that whatever was on the rock has moved into the tank and wood somewhat.
However we have also had a few of the plants shed some leaves. Not a lot but maybe a leaf every day or two. And the bogwood is organic itself so might be decaying a bit too.
Just wondering what to do now?
Similar threads
- Replies
- 7
- Views
- 208
- Replies
- 5
- Views
- 363